No more explosions! First non-flammable lithium ion battery may make smartphones safer

We read regularly about that epic moment in a smartphone owner’s life, the device explodes in a pocket, or while charging. Sometimes, these incidents cause serious damage to property or harm to the user.
There are a number of reasons why these things happen, not the least of which is user negligence. However, lithium-ion batteries are highly flammable and these incidents are not limited to smartphones or other consumer electronics.
Boeing’s 787 Dreamliner aircraft have been grounded several times over the past year due to problems with that aircraft’s lithium-ion battery packs. Given the other efficiencies the Li-ion batteries offer however, they are here to stay.
When you think about it though, battery advances do not seem to be keeping pace with other sectors of technology. It would be nice if we could start seeing higher capacities in ever shrinking packages. That progress seems to be coming rather slowly, but there is another front where battery technology could use some help, flammability.
Li-ion batteries are explosive for two primary reasons, they are pressurized and the electrolyte that carries the charge is flammable. Since we are waiting for more power, making the current crop of batteries less flammable is an obvious goal worth reaching.
Enter researchers at the University of North Carolina at Chapel Hill. These scientists claim they have developed the first non-flammable lithium-ion battery. This was achieved by replacing the electrolye’s flammable organic solvent with an industrial lubricant known as perfluoropolyether (PFPE).
PFPE is usually used in maritime applications to keep sealife from sticking to the bottom of large boats. Joseph DeSimone, the research lead in this development, determined that dissolving a lithium salt in PFPE did the trick and it resulted in a battery that not only was not flammable, but indicated possibly longer battery life for good measure.
“These electrolytes not only are completely nonflammable, but they also exhibi unprecedented high transference numbers and low electrochemical polarization, indicative of longer battery life,” according to DeSimone’s summary.
Research must continue to see if this discovery can be put to practical use, and if such a design can withstand consistent charging and discharging like any other consumer device. Then there is also the challenge of finding a way to mass produce these new batteries.
For those that may think that is an easy task, just look at what lithium-ion batteries endure before they explode in the video below.
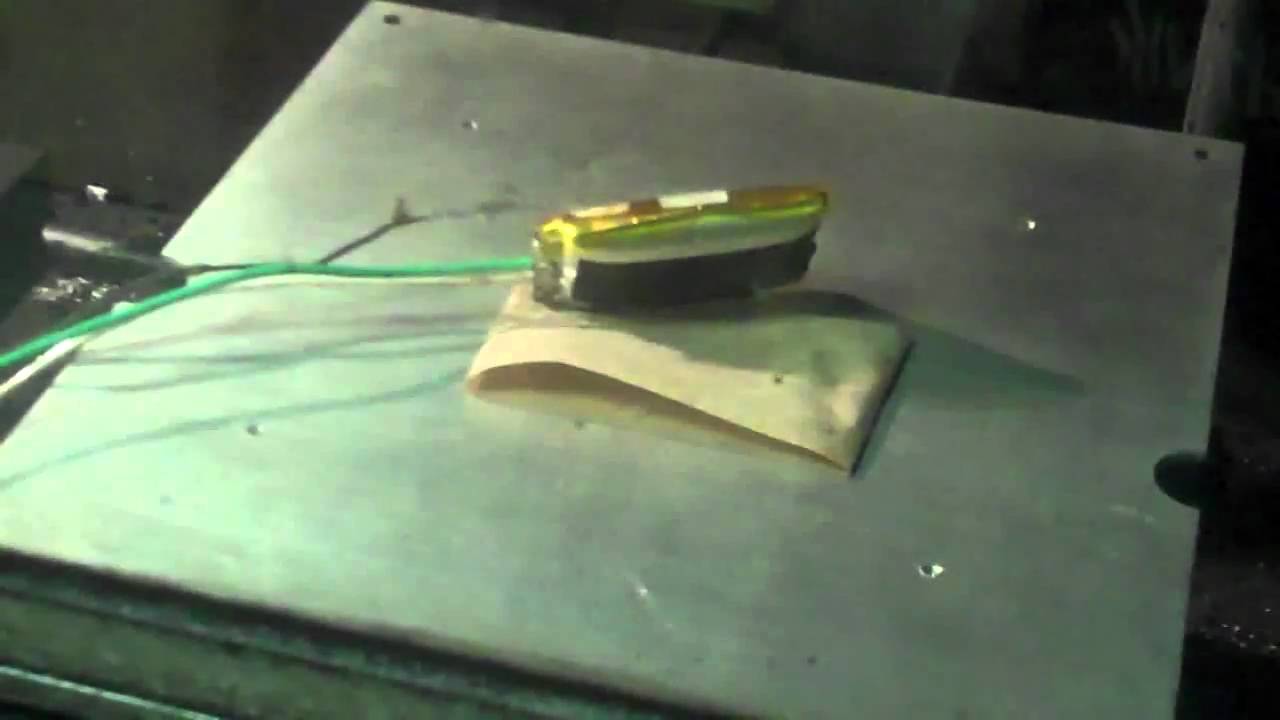
source: ExtremeTech
Boeing’s 787 Dreamliner aircraft have been grounded several times over the past year due to problems with that aircraft’s lithium-ion battery packs. Given the other efficiencies the Li-ion batteries offer however, they are here to stay.
Li-ion batteries are explosive for two primary reasons, they are pressurized and the electrolyte that carries the charge is flammable. Since we are waiting for more power, making the current crop of batteries less flammable is an obvious goal worth reaching.
PFPE is usually used in maritime applications to keep sealife from sticking to the bottom of large boats. Joseph DeSimone, the research lead in this development, determined that dissolving a lithium salt in PFPE did the trick and it resulted in a battery that not only was not flammable, but indicated possibly longer battery life for good measure.
“These electrolytes not only are completely nonflammable, but they also exhibi unprecedented high transference numbers and low electrochemical polarization, indicative of longer battery life,” according to DeSimone’s summary.
For those that may think that is an easy task, just look at what lithium-ion batteries endure before they explode in the video below.

source: ExtremeTech
Follow us on Google News













Things that are NOT allowed:
To help keep our community safe and free from spam, we apply temporary limits to newly created accounts: